Discovery of heat
Heat is not a substance but rather energy that can be gained or lost. Early scientist thought that heat could just evolve and naturally flow between hot and cold objects. Benjamin Thompson (Count Rumford disproved this by showing that as you drilled the hole in cannon barrels under water the temperature of the water rose. He concluded that the temperature rise in the water was due to the work that was being done on the cannon and that is was not just flowing by itself. He concluded that heat must be a form of energy.
What is thermal energy?
Thermal energy is the energy portion of a system that increases with its temperature.
Thermal energy is the total kinetic energy of atoms or molecules in a body.
What is the TRANSFER of thermal energy?
Heat
What causes transfer of thermal energy?
It is the hot mass or anything else different in temperatures between objects that allows thermal energy to flow from a region of higher temperature to a region of lower temperature
.
!! The greater the temperature difference, the greater the heat flow

Applications:
Ж A cold spoon feels hot after putting it in in hot milo.
Ж Your hand near the iron feels warmth
[ take note : temperature not = to thermal energy ]
Temperature is a measure of thermal energy. It tells us how hot or cold an object is .
How is thermal energy transferred ?
In this video, it summarises the 3 methods of heat conduction.
( please refer to first video you see) - according to date!
Firstly, conduction is shown through using a rod as conduction is the kind of heat transfer that do not require the medium to move. And how the ord gets heated up and conduct the heat to the hand.
For convection, it is by the heat felt above the flame, by the movement of the air above the flame that helps transfer this heat.
Lastly, radiation helps transfer heat by infared waves that do not require a medium.
This shows how a stick gets heated up by conduction. Take note of the colour difference that depicts the heat.
As seen from above, 3 mechanisms are in charge of transferring heat energy :
1. Conduction
2. Convection
3. Radiation
Now, what do this three methods aim to achieve ?
- A thermal equilibrium (no net movement of thermal energy).
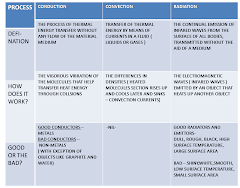
Conduction
Is the process of thermal energy transfer without any flow of the material medium.
Process
1. When a part is supplied with thermal energy, the molecules at this area gain kinetic energy. They vibrate faster and collide with the neighbouring particles
2. As molecules collide, there is kinetic energy transfer. The less energetic molecules gain kinetic energy, vibrate faster and collide with other less energetic molecules in the colder part of the object
3. Process continues until heat energy from the hotter part spreads throughout the colder area
Solids are better than liquids and gases because the molecules are much closer together. Since the atoms are closer together, solids conduct heat better than liquids or gasses. This means that two solid materials in contact would transfer heat from one to the other better than a solid in contact with a gas or a gas with a liquid.
Solids can either be metals or non-metals. Metals are GOOD CONDUCTORS because they have FREE ELECTRONS which are not possessed by non-metals.
When a metal is heated, free electrons in the metal move faster. These fast-moving energy carrying electrons gain kinetic energy, diffuse and spread to cooler parts (by colliding with them)thus transferring kinetic energy. Heat is transferred.
A good example would be copper, and how heat is transferred in it.
When the copper rod is heated, the free electrons in the copper gain kinetic energy and it will move faster as a result. These fast-moving electrons then diffuse or spread into the cooler parts o the metal. In the process, they collide with the atoms in the cooler parts of the metal and transfer their kinetic energies to them. This explains why good conductors like metals are capable of transferring thermal energy much faster than insulators ( ie non-metals).
 * water and air at different rates
* water and air at different rates
This diagram may be a bit unclear. But it shows two areas of conduction. The one above with super large molecules are normal conduction, and if we zoom in ( blue circle ) we can see that they bump into one another and that is how heat is transferred. But for metals, there is free electron diffusion that hits against the molecules allowing them to gain heat, and this is displayed by the green circles hitting against the brown particles.
Examples of everyday applications:
All metals are good conductors of heat, eg cooking pans, kettles, electric iron are made of copper, aluminium or steel. For example - pipes - steam pipes and boilers to conduct away heat, thus preventing overheating.
Plastic and wood are poor conductors but good insulators, eg handles of cooking utensils are made of plastic or wood.
Air is a very good insulator. A layer of trapped air can slow down the conduction process. Due to the large spacings the molecules have in between one another.
The air (being a poor conductor of heat) trapped in and between our clothes and blankets slows down heat loss, and therefore keep us warm.
In the same way, the air trapped in fur and feather keeps animals warm.
A refrigerator has insulation material around itself. The insulation largely reduces the amount of heat transfer from the outside warmer temperature. Similarly, double-glazed windows reduce heat into the house.
Common Experiments

First of all, take a test-tube and fill three'-fourths of it with water. Now take an ice cube and coil the wire around it ( prevents ice from floating). Drop it in the test-tube. Normally, the ice floats on water, but because of the wire, it will sink to the bottom.
Now take a candle and light it. Tilt the test-tube a little and bring its mouth near the flame to heat the upper surface of water. You'll be surprised to see that the water at the upper surface gets heated and starts converting into steam, while the ice lying at the bottom remains as it is.
WHY IS IT SO?
We must understand that water is a bad conuductor of heat, therefore heat cannot travel so efficently to the ice. We may also see that convection currents are present - butonly on the top. Thus, all the hot water boiled remains and the top and does not go to the bottom of the test tube as hot water = lower density = rises and cooled water= higher density=sinks. Therefore the ice remains intact and unmelted.
Above table mainly shows how good the metals are in conducting heat. Only FYI. Above picture shows a lamp invented by Sir Humphry. The meshed metal conducts heat away quickly to prevent any explosive gas from overheating, and thus avoiding an explosion.
Above picture shows a lamp invented by Sir Humphry. The meshed metal conducts heat away quickly to prevent any explosive gas from overheating, and thus avoiding an explosion.
The brown areas are made up of copper and these lamps are used in mines.
There, they are safe and would not ignite an explosion.
WHY IS THIS SO?
The copper wires help to conduct the heat, so little heat is lost through other means and instead, most are conducted within the copper wire as copper is a good conductor of heat, so less heat is transferred to the surrounding environment.
What is the worst conductor ?
VACUUM!
Why does a metal surface feel colder than a wooden one ?
Heat is transferred faster from your hand away from the surroundings in the case of the metal surface instead of the wooden one, thus making your hand feel colder, since the metal surface is a better conductor.
CONVECTION
Convection is the transfer of thermal energy by means of a current which invokes a bulk movement
Process
When thermal energy is supplied to a region of a fluid, it EXPANDS.
This region is now less dense than the surrounding fluid and therefore RISES.
The other cooler regions of the fluid, being denser, SINK to replace the less dense fluid (at a larger distance versus conduction).
This movement of the fluid due to a DIFFERENCE IN DENSITY sets up a convection current.
Shows a convection current in the room that uses a heater to create the density difference

Above shows convection currents in liquids.
Gases expand more than liquids, resulting in a stronger and faster convection.
Examples of everyday applications:
Car engines
Car engines are cooled by convection currents in the water pipes. Water is a very good substance to carry the unwanted heat away from the engine to the radiator. Te radiator is a heat exchanger where the hot water gives up its energy to the air.
When a car engine has been running for a long time, a lot of thermal energy is produced. It is necessary to cool the engine so that it does not over-heat. The engine is surrounded by a water jacket. When the water in the water jacket gets heated, it flows into copper tubes which include many cooling fins. A fan causes air to flow past these tubes and cool the water in them. The cooled water flows down, back into the engine through a hose at the bottom. A water pump is normally employed to facilitate the flow of convection currents back into the jacket.
Monsoons
The hot land heats up the air above. Cooler air from the ocean blow towards land to take the place of the hot air. The cooler air contains water vapour that condenses on clouds, thus causing rain.
Below shows a simple domestic hot-water system. Convection currents rive the hot water up from the boiler to the storage tank while cold water flows down to the boiler.
The operation of a hot water system for a house is based on the principle of convection. The system consists of a boiler, a water storage tank and a cold tank inter-connected by pipes arranged as shown in the figure. Convection currents drive hot water from the top of the boiler into the hot water storage tank. Cold water from the sotrage tank is drawn down to the boiler, where it in turn becomes heated.

Sea and Land Breezes
The sea and land breezes over coastal areas are natural convection currents.
During the day, the sun heats up the land much faster than the sea (partly due to efficient conduction in the ground).
On the other hand, water is a poor conductor of thermal energy.
Convection cannot take place efficiently in the sea since only the surface water is being heated, and not the bottom layer.
Subsequently the temperature of the sea water takes a long time to rise.
All this while, the air above the land is being heated.
It expands and rises. Cool air above the sea (being of lower temperature) then moves inland to take its place.
The result is a sea breeze.
At night, the opposite happens.
The sea temperature drops slowly, as compared to the land which cools very rapidly,.
The sea is now warmer than the land.
The warm air above the sea rises ad the cool air above the land moves out to take its place and we have a land breeze.
Sample Question:
Typical ones woould be like:
Why is the freezer and the top of the fridge?
Why is the air-con above, high in the ceiling?
OR
Why is the heating element in a kettle at the bottom?
As such, only need to apply your understanding on convection:
It is placed on top to faciliate convection currents whereby - hot water/air rises and cold air sinks. In this case, the hot air would rise up and the freezer would help to cool the air which then would sink due to the increase in density that makes it heavier than its surrounding. This movement would displace the heated air at the bottom to rise up ( also because of its small density value ). This process repeats itself, thus effficently cooling the air in the frigde.
a little long :D
How does a bottom-freezer refridgerator work ?
Bottom-freezer refrigerators have many benefits that equal or surpass those of models that feature different designs. For instance, of the three major refrigerator-freezer configurations -- top-freezer, side-by-side and bottom-freezer -- the bottom-freezer models are generally the most energy efficient. This is because warm air rises and in these models, the warmer part of the appliance is located on top.
The concept of convection still applies here, but in a different form. Cool eh?
RADIATION
Radiation is the means by which energy reaches us from the Sun. As there is a vacuum between the Earth and the Sun, conduction and convection cannot take place. The Sun’s energy travels to Earth as electromagnetic waves at the speed of light. Radiation is thus the transfer of thermal energy by electromagnetic waves. These waves constitute to a part of the electromagnetic spectrum, and are known as infra-red radiation. When absorbed, the energy of the waves transforms into thermal energy of the receiving body.
The hotter an object is, the more energy it radiates. For example, the radiation from a red hot iron or grill is more easily felt than the radiation from a hot cup of tea.
The rate of energy transfer by radiation is affected by:
(a) Surface temperature
(b) colour and texture of the surface
(c) Surface area
In really detailed details :
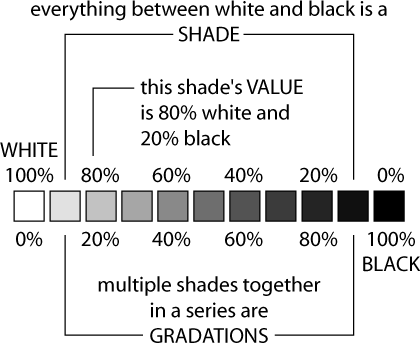
1 Colour and texture of the surface
Dull black rough surfaces are better absorbers of infrared radiation than shiny, white , smooth surfaces ( can see it as it reflecting heat ). The diagram shows the shades of black.
2 Surface temperature
The rate of infrared radiation also depends on the surface temperature. The higher the temperature of the surface of the object relative to the surrounding temperature, the higher will be the rate of infrared radiation.
Please note that the temperature of an object does not determine the speed at which radiation takes place. It only affects the wavelength and amt of infra-red radiation emitted.
The rate if radiation is referred as the amount of heat transferred by radiation, and NOT the speed.
3 Surface area
The third factor which affects the rate of radiation emission and absorption is the surface area of the object. If we compare two objects of the same mass and material, but with different surface areas, the object with the larger surface area would emit infrared radiation at a higher rate.
Applications of radiation
Houses in hot countries are often painted white to keep them cool. Factory roofs are sometimes coated with aluminium paint. This reduces the absorption of radiation during the day, and cuts down the emission of radiation during the night. So a fairly steady temperature is maintained within the factory.
Solar control films are usually made of polyester layers combined with special ultraviolet radiation-absorbing dyes and highly reflective metals. They allow light to pass through windows of cars and buildings while keeping the infra-red radiation out.
Conversely, solar panels are deliberately painted dull black in order to absorb as much radiation from the Sun as possible.
Polar bears
The polar bear is a natural example of one that increases heat transfer to itself. Its skin is black in colour as it is a good absorber of heat. Thus able to make It feel warm.
It has transpoarent fur, which is seemingly white, It helps to trap the heat absorbed ( white is a bad emitter of heat. This efffectvely keeps it warm.
Experiments regarding Radiation
that can possibly be qns you see in exams and so on...
A body’s temperature rises when it absorbs radiation. We will now see how the surface will affect its ability to absorb heat through radiation.
Here, it shows two tin cans and how radiation is relative to the temperture that the objects are in. As seen, in the sunny area, the black tin can would be of a higher temperature as it is a good absorber. It cool places however, when hot water is poured into this cans, it loses heat to the surroundings, black loses heat the fastest, thus leaving the white one to be hotter.
( i cant flip it :(
Two tin can lids are taken and one of them is painted dull black on the inside surface while the other one is left shiny. Corks are stuck on the outside of each lid with candle wax. A heater is placed midway between the lids. Observation shows that the wax on the blackened lid melts in a short time, and the cork on it falls off. The shiny lid, however, remains fairly cool and the wax on it remains unmelted.
A green house is made up of glass. It takes solar energy form the sun and converts it into thermal energy. You can easily observe this happening in your day to day life. On a hot sunny day, when you park your car in sunlight with the windows rolled up. The solar energy passes through the window and heats up the inner atmosphere of your car. As a result, everything in the car becomes warm. This happens because the infra red rays are of a shorter wavelength before they touch the window.
As they pass through the glass, they become of a longer wavelength. Infra red rays of a longer wave length cannot escape. As a result they get absorbed by the objects surrounding them. This is how a green house creates an ideal setting to grow plants. Plants get enough energy and sun light to absorb and grow.
IMPORTANT APPLICATIONS THAT HAS ALL 3 METHODS OF HEAT TRANSFER.
1) Vacuum Flask
A vacuum (thermos) flask is designed to keep hot liquids hot, and cold liquids cold. Essentially, we need to reduce the transfer of thermal energy by conduction, convection and radiation.
The vacuum flask consists of a double-walled glass container, with a vacuum between the walls. Both walls are silvered on the vacuum side. The container is supported on foam plastic which is a poor conductor of thermal energy.
No thermal energy can enter or leave the flask by conduction or convection across the vacuum. The inner silvered surface reflects radiation from hot fluids back into the flask. The outer silvered surface reflects radiation in the external surroundings away from the flask. The foam plastic support and the plastic cap also minimise the thermal energy transmitted by conduction through the thin glass walls of the flask. Lastly, the plastic cap stops convection and evaporation.
2) Heat Sink for microprocessor

When a microprocessor chip gets overheated, it will be damaged. To overcome the problem of overheating, conduction, convection and radiation are used to remove as much heat as possible from the device to keep it cool.
Conduction - the heat sink is made from a good conductor, eg aluminium. It is fastened firmly to the chip. This allows thermal energy to be conducted effectively from the chip to the heat sink.
Convection - the surface area of the metal in contact with air is increased by shaping the heat sink into many flat layers. Once the thermal energy is conducted to the air, convection will bring the thermal energy away. A fan is usually mounted on the heat sink to increase the rate of convection.
Radiation - the increased surface area of the heat sink increases the rate of energy transfer by radiation.
3) BULB
Thermal conduction takes place along the supporting wires and the hot filament.
One convection current can be set up in the inert gas inside the bulb and another is set up in the air surrounding the bulb, as can be seen by the purple arrows.
The very hot filament heats up the inert gas near it and this gives rise to a lower density of the gas. The warm gas rises and the cooler gas sinks, setting up the first convection current.
Due to radiation (green arrows), the air at the top of the bulb will be warmer compared to the air at the bottom of the bulb. The warm air rises and cool air sinks, setting up the second convection current.
EXTRA KNOWLEDGE :D
How does a freezer work ?
A refrigerator and a freezer both work in a similar way except the temperature of the refrigerator is kept a little above the freezing point to avoid the decay of food products. A freezer maintains the temperature of up to -18 degrees, it keeps food frozen. Every refrigerator has a compressor and uses a compression cycle in order to keep the inside of the refrigerator cool. A refrigerant called "Freon" is used in this cycle. This agent is in the form of a vapour and it enters the compressor at its boiling point, where it is compressed.
At this point, the vapour is superheated and is condensed in to a saturated liquid. It then goes to an expansion valve where the pressure is decreased and the vapour evaporates. This liquid is not cool and partially vaporized. It travels through the coil that you see behind your refrigerator, where a fan totally vaporizes the refrigerant and makes it enter the freezer where it keeps the food frozen.

Ok. How are pizzas kept warm ? look above.
The heated pouch replaces the usual vinyl (which is a really bad conductor of heat) insulated pouches, currently used for delivery for Pizza Huts. The heated pouch, a tremendous success in several countries abroad has thermal heating pads placed within.
The hi-tech thermal pads placed inside the pouches will control heat loss and maintain the temperature at 70-75 degrees centigrade which is considered to be the ideal temperature for serving food.
In standard deliveries the heat-loss is usually from the pizza carton into the pouch which leads to a loss of 10-15 degrees in temperature, a variation that makes all the difference between just hot to sizzling hot.
If the pizza is hot on arrival, the special heat-sensitive sticker will transform from its original black to white, revealing the word “hot”.If you did not notice, the pizza boxes have a cardboard like, uneven piece. Between each "hill" (uneven folds) air is trapped. As air is a bad conductor of heat, it effectively keeps the pizza warm. Notice what material is the pizza box made of?